Local Anesthetic Systemic Toxicity (LAST)
Kristin Barkley, DNP, CRNA
Local Anesthetic Systemic Toxicity (LAST)
- LAST is a potentially fatal complication of local anesthetic (LA) administration causing central nervous system (CNS) and/or cardiovascular (CV) toxicity.
- Mechanism: at toxic levels, LAs depress voltage-gated sodium (Na+), potassium (K+), and calcium (Ca2+) channels in excitable tissues of the CNS and CV systems.
- Incidence varies by block type and setting; registry estimates ~0.3 per 1,000 peripheral nerve blocks, with neuraxial generally lower. A notable proportion occur outside the hospital.
- Most LAs are lipophilic and highly protein-bound; longer-acting, more lipophilic agents tend to be more cardiotoxic.
Procedure Guide
Types of Blocks (risk/order varies by site & vascularity)
- Common sites/techniques (approx. frequency in many practices, not exhaustive): circumcision; neuraxial; upper extremity; head & neck; topical/infiltration; transversus abdominis plane (TAP); intravenous regional anesthesia.
- Higher systemic absorption risk with more vascular sites; consider tailored dosing and vigilance by block site.
Local Anesthetics – Mechanism of Action
- LAs block voltage-gated Na+ channels from the intracellular side, preventing depolarization and action potential propagation → analgesia/anesthesia.
- Shorter-acting agents are generally less cardiotoxic than longer-acting agents.
- Greater potency ↔ higher lipophilicity and protein binding (e.g., bupivacaine > lidocaine for cardiotoxic risk).
- Large-volume blocks are higher risk if dosing/absorption are not carefully controlled.
- pKa & pH: lower pKa → more nonionized fraction → faster membrane transit.
Clinical Presentation – CNS (most common initial)
- Subjective prodrome: agitation, tinnitus, circumoral numbness, blurred vision, metallic taste.
- Progression: excitatory → depression (muscle twitching, seizures, unconsciousness).
- Advanced: respiratory arrest; CV involvement may follow.
Clinical Presentation – Cardiovascular
- Can be the first/only sign in severe LAST.
- Typical sequence (most to least frequent): dysrhythmias, conduction delay, cardiac arrest, bradycardia/hypotension.
Presentation Mix & Onset
- Approximate distribution at recognition: CNS-only, CV-only, or combined presentations.
- Onset: ~75% within 10 minutes of injection; may be delayed with infusions/continuous techniques.
Block Site & Relative Risk of Systemic Absorption
- Higher risk with intercostal and paravertebral (highly vascular) > caudal/epidural > interfascial plane blocks (abdominal wall) > psoas compartment > sciatic > brachial plexus/cervical (varies) > subcutaneous/intra-articular.
Exparel (Liposomal Bupivacaine) – Compatibility Notes
- May be admixed only with bupivacaine HCl; total bupivacaine HCl dose should not exceed a 1:2 ratio relative to Exparel.
- Do not admix with non-bupivacaine LAs (e.g., lidocaine) due to rapid release; Exparel may follow lidocaine after ≥20 minutes.
Lipid Emulsion – Rationale
- “Lipid sink/shuttle”: sequesters LA away from heart/brain for subsequent metabolism/elimination.
- May also exert direct cardiotonic and metabolic effects (Na+ channels, fatty-acid utilization, mitochondria).
- Rare complications: pancreatitis, DVT—monitor labs post-treatment.
SCOPe Guide
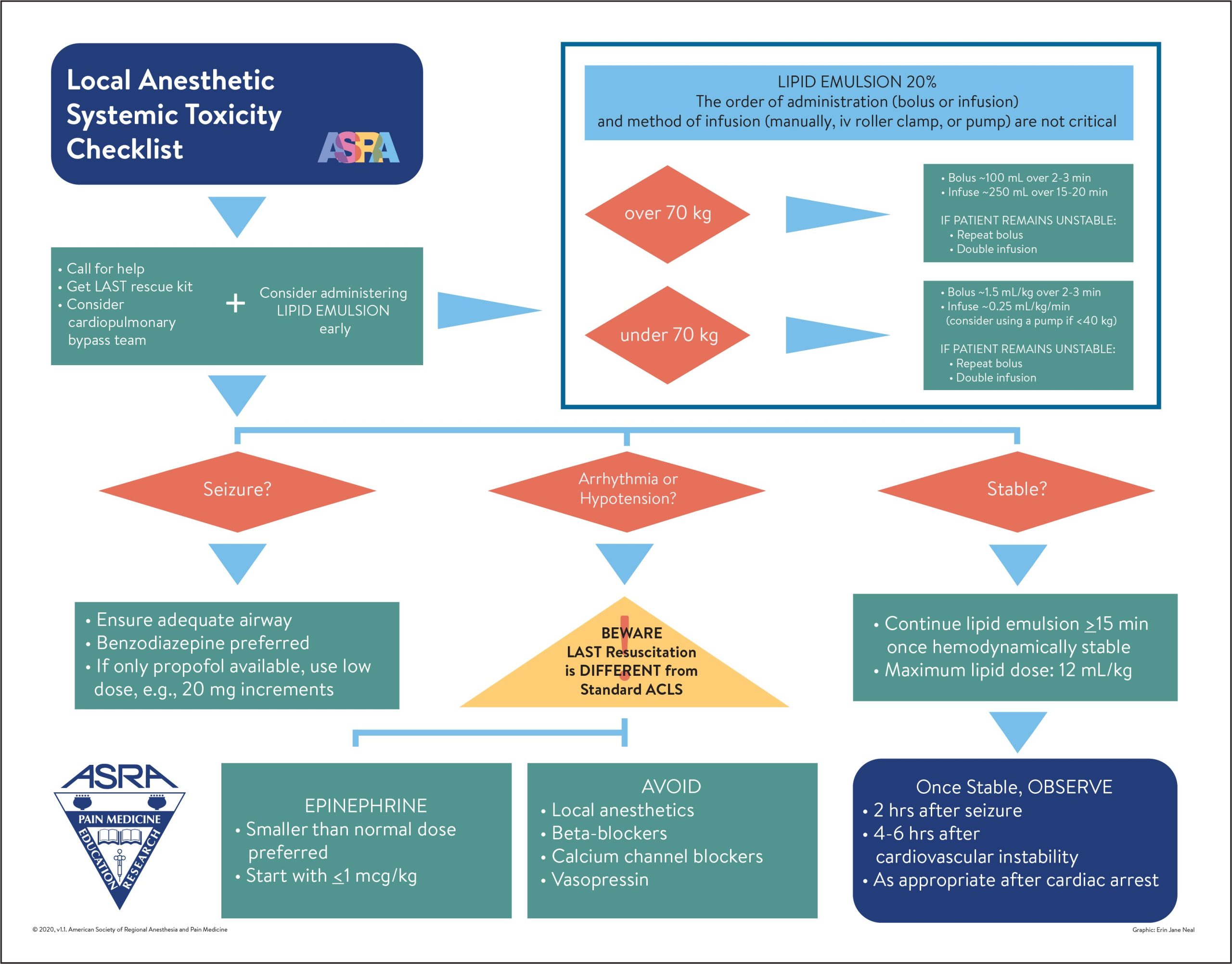
Strategies – Treatment (ASRA Checklist-guided)
Initial
- Call for help.
- Airway & Breathing: 100% O2; manage ventilation/oxygenation early.
- Seizure suppression: benzodiazepines preferred; if only propofol is available, use small, titrated doses.
- BLS/ACLS with LAST-specific modifications (see below).
20% Intravenous Lipid Emulsion (use lean/ideal body weight if applicable)
- <70 kg: Bolus 1.5 mL/kg over 2–3 min → infusion 0.25–0.5 mL/kg/min.
- Repeat bolus up to 3× for persistent CV collapse; consider doubling infusion if unstable.
- Continue infusion ≥10 min after achieving hemodynamic stability.
- Recommended upper limit over first 30 min ≈ 12 mL/kg.
- ≥70 kg (adult): Bolus 100 mL over 2–3 min → infusion 200–250 mL over 10–20 min.
AVOID / MODIFY
- Avoid: vasopressin, beta blockers, calcium channel blockers, additional local anesthetic.
- Epinephrine: use smaller doses; start <1 mcg/kg per dose during resuscitation.
- Avoid propofol as an anticonvulsant in CV instability.
Escalation
- Prepare for cardiopulmonary bypass/ECMO when available for refractory collapse.
Clinical Optimization – Prevention & Detection
- Preparation: When using LAs, stock a LAST kit and post the treatment algorithm where blocks are performed.
- Risk reduction: lowest effective dose; frequent aspiration; test doses; incremental dosing; ultrasound guidance; keep patient lightly sedated to allow feedback.
- Patient factors increasing risk: advanced age; hepatic/renal disease; conduction abnormalities; low plasma protein (↑ free drug); metabolic/respiratory acidosis; heart failure; pregnancy; mitochondrial disorders.
- Monitoring: observe during and ≥30 minutes after injection; consider LAST with any new neurologic changes or hemodynamic instability after regional anesthesia.
- CV progression cue: early hyperdynamic signs (tachycardia, hypertension, ventricular ectopy) can precede hypotension, conduction block, bradycardia, VT/TdP/VF, or arrest.
Pearls
- Know where lipid emulsion is stored before starting blocks; check expiration volume.
- Initiate the LAST protocol early and titrate to clinical severity and speed of progression.
- Use lean/ideal body weight for dosing when appropriate.
- High-risk patients/blocks warrant extra vigilance and extended observation.
Media
Copyright © 2020 by the American Society of Regional Anesthesia and Pain Medicine.
References
- ASRA. Checklist for Treatment of Local Anesthetic Systemic Toxicity (2020).
- ASRA. LAST Checklist PDF (2020 update).
- UpToDate: Local anesthetic systemic toxicity.
- Elisha S, Heiner JS, Nagelhout JJ. Nurse Anesthesia. 7th ed. Elsevier; 2023.
- EXPAREL FDA Label (compatibility & timing guidance).
Media Attributions
- local-anesthetic-systemic-toxicity-rgb

