03-2 Heredity & Prenatal Development
Alisa Beyer and Julie Lazzara


Photos Courtesy of Will Hastings (Left), Mamma Loves (Center) and MIKI Yoshihito (Right)
Objectives:
At the end of this chapter, you will be able to…
- Define gene, chromosome, and gamete.
- Distinguish between mitosis and meiosis, genotype and phenotype, homozygous and heterozygous, and dominant and recessive.
- Question the assertion that human traits are genetic. Define genotype-environment correlations and genotype-environment interactions, and define epigenetics.
- Differentiate between genetic disorders and chromosomal abnormalities. Describe Trisomy 21.
- Describe the function of genetic counseling.
- Describe human development during the germinal, embryonic, and fetal periods and differentiate between the three periods of development.
The objectives are indicated in the reading sections below.
Introduction
In this chapter, we will begin by examining some of how heredity helps to shape the way we are. We will look at what happens genetically during conception, and describe some known genetic and chromosomal disorders. Next, we will consider what happens during prenatal development, including the impact of teratogens. We will also discuss the impact that both partners (e.g., mother and father) have on the developing fetus. Next, we will present the birth process and some of the complications that can occur during delivery. Before going into these topics, however, it is essential to understand how genes and chromosomes affect development.
Heredity: The Epigenetic Framework (Ob 3)
Nature and Nurture
In this lesson, we will look at some of how heredity helps to shape the way we are. We will look at what happens genetically during conception and take a brief look at some genetic abnormalities. Before going into these topics, however, it is essential to emphasize the interplay between heredity and the environment. Why are you the way you are? As you consider some of your features (height, weight, personality, being diabetic, etc.), ask yourself whether these features are a result of heredity or environmental factors, or both. Chances are, you can see the ways in which both heredity and environmental factors (such as lifestyle, diet, and so on) have contributed to these features. For decades, scholars have carried on the “nature/nurture” debate. For any particular feature, those on the “nature” side would argue that heredity plays the most important role in bringing about that feature. Those on the “nurture” side would argue that one’s environment is most significant in shaping the way we are. This debate continues in questions about what makes us masculine or feminine (Lippa, 2002), concerns about vision (Mutti, Kadnik, & Adams, 1996), and many other developmental issues. Most scholars agree that there is a constant interplay between the two forces. It is difficult to isolate the root of any single behavior as a result solely of nature or nurture, and most scholars believe that even determining the extent to which nature or nurture impacts a human feature is difficult to answer. Almost all human features are polygenic (a result of many genes) and multifactorial (a result of many factors, both genetic and environmental). It is as if one’s genetic make-up sets up a range of possibilities, which may or may not be realized depending upon one’s environmental experiences. For instance, a person might be genetically predisposed to develop diabetes, but the person’s lifestyle may help bring about the disease.
The Epigenetic Framework
Gottlieb (1998, 2000, 2002) suggests an analytic framework for the nature/nurture debate that recognizes the interplay between the environment, behavior, and genetic expression. This bidirectional interplay suggests that the environment can affect the expression of genes just as genetic predispositions can impact a person’s potentials. Moreover, environmental circumstances can trigger symptoms of a genetic disorder. For example, a person who has sickle cell anemia, a recessive gene linked disorder, can experience a sickle cell crisis under conditions of oxygen deprivation. Someone predisposed genetically for type two diabetes can trigger the disease through poor diet and little exercise.
The Human Genome Project (Ob 1, Ob 2)
The Human Genome Project is an internationally funded effort to map the locations of human genes and understand the role these genes play in development, health, and illness. Genes are segments of chromosomes (46 strands of a chemical substance called DNA that is contained in the nucleus of each normal human cell) that vary in length. There are an estimated 25,000 to 30,000 genes on each chromosome; a number far below the estimate of 100,000-150,000 held before the work of the Human Genome Project.
Understanding the role of genes in health and illness can bring about both harm and good (Weitz, 2007). A person who knows that they are at risk for developing a genetic disorder may be able to adopt lifestyle practices that minimize the risk and a person who discovers that they are not at risk may find comfort in knowing that they do not have to fear a particular disease. However, a person who finds out that they are at risk and there is nothing that can be done about it may experience years of fear and anxiety. Moreover, the availability of genetic testing may be more widespread than the availability of genetic counseling which can be very expensive. The possible stigma and discrimination that those with illness or at risk for illness must also be considered. In light of the high costs of health insurance, many companies are starting to offer benefits contingent on health assessments and lifestyle recommendations; and continued coverage depends on an employee following these recommendations. So, a smoker may have to pay a higher premium than a non-smoker, or a person who is overweight may be required to engage in a program of exercise and be monitored for improvement. What if a person finds out that they carry the gene for Huntington’s disease (a neurological disorder that is ultimately fatal) which may surface when a person reaches their 40s? The impact this knowledge will have on health care remains unknown.
 Who should know what is on your genome? Do you think this information should be shared between mates? What about employers? What would be the advantages and disadvantages?
Who should know what is on your genome? Do you think this information should be shared between mates? What about employers? What would be the advantages and disadvantages?
The Female Reproductive System
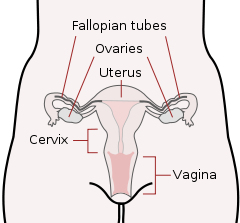
Photo Courtesy of GTECH
The cells used in sexual reproduction are called gametes. There are two types of sex cells or gametes involved in reproduction: the male gametes or sperm and female gametes or ova. The male gametes are produced in the testes in a process called spermatogenesis which begins at about 12 years of age. The female gametes or ova which are stored in the ovaries are present at birth but are immature. Each ovary contains about 250,000 (Rome, 1998) but only about 400 of these will become mature eggs (Mackon & Fauser, 2000). Beginning at puberty, one ovum ripens and is released about every 28 days, a process called oogenesis.
Chromosomes contain genetic information from each parent. While other normal human cells have 46 chromosomes (or 23 pair), gametes contain 23 chromosomes. In a process called meiosis, segments of the chromosomes from each parent form pairs and genetic segments are exchanged as determined by chance. (For normal human cells the process is called mitosis as the cell’s nucleus making an exact copy of all the chromosomes and splitting into two new cells). Because of the unpredictability of this exchange, the likelihood of having offspring that are genetically identical (and not monozygotic twins) is one in trillions (Gould & Keeton, 1997).
Determining the Sex of the Child (Ob 4)
Twenty-two of those chromosomes from each parent are similar in length to a corresponding chromosome from the other parent. However, the remaining chromosome looks like an X or a Y. Half of the male’s sperm contains a Y chromosome, and half contain an X. All of the ova contain two X chromosomes. If the child receives the combination of XY, the child will be genetically male. If it receives the XX combination, the child will be genetically female.
Monozygotic and Dizygotic Twins
Monozygotic twins occur when a single zygote or fertilized egg split apart in the first two weeks of development. The result is the creation of two separate but genetically identical offspring. About one-third of twins are monozygotic twins (identical twins). Are you an identical twin?
Sometimes, however, two eggs or ova are released and fertilized by two separate sperm. The result is dizygotic or fraternal twins. About two-thirds of twins are dizygotic. These two individuals share the same amount of genetic material as would any two children from the same mother and father. Older mothers are more likely to have dizygotic twins than are younger mothers and couples who use fertility drugs are also more likely to give birth to dizygotic twins. Consequently, there has been an increase in the number of fraternal twins in recent years (Bortolus et al., 1999).
What are the other possibilities? Various degrees of sharing the placenta can occur depending on the timing of the separation and duplication of cells. This is known as placentation. Here is a diagram that illustrates various types of twins.
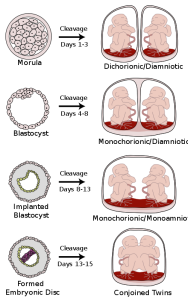
Figure caption: cellular changes in the formation of different types of twins. In som cases twins share the same amniotic sac and other cases they are in separate amniotic sacs with individual placentas. Photo Courtesy of WikiCommons
Genotypes and Phenotypes (or why what you get is not always what you see) (Ob 2)
The word genotype refers to the total of all the genes a person inherits. The word phenotype refers to the features that are expressed. Look in the mirror. What do you see, your genotype, or your phenotype? What determines whether or not genes are expressed? Actually, this is quite complicated (Berger, 2005).
Because genes are inherited in pairs on the chromosomes, we may receive either the same version of a gene from our mother and father, that is, be homozygous for that characteristic the gene influences. If we receive a different version of the gene from each parent, that is referred to as heterozygous. In the homozygous situation, we will display that characteristic. It is in the heterozygous condition that it becomes clear that not all genes are created equal. Some genes are dominant, meaning they express themselves in the phenotype even when paired with a different version of the gene, while their silent partner is called recessive. Recessive genes express themselves only when paired with a similar version gene.
Table Examples of Single-Gene Dominant-Recessive Inheritance
| Dominant | Recessive |
|---|---|
| window’s peak | no widow’s peak |
| freckles | no freckles |
| unattached ear lobe | attached ear lobe |
See more examples here |
|
Source: Genovesi, Blinderman, & Natale, 2022
Geneticists refer to different versions of a gene as alleles. Some single-gene dominant traits include having facial dimples, normal vision, and dark hair. Some single-gene recessive traits include red hair and being nearsighted. Sometimes the dominant gene does not entirely suppress the recessive gene; this is called incomplete dominance. An example of this is hair being curly or straight. If you have straight hair you have SS, curly hair is CC and Wavy hair is CS.
Most characteristics are not the result of a single gene; they are polygenic, meaning they are the result of several genes. Consider eye color. Eye color is influenced mainly by two genes, with smaller contributions from several others. People with light eyes tend to carry recessive alleles of the major genes; people with dark eyes tend to carry dominant alleles. Also, the dominant and recessive patterns described above are usually not that simple either. The words dominant and recessive of polygenes are not truly dominant and recessive. Some features follow the additive pattern which means that many different genes contribute to an outcome. Height, weight, skin tone, and intelligence are examples of polygenic inheritance. Take for example, skin, where an individual would have a combination of 3 gene pairs (e.g., AABBCC). Off spring then would have combinations of each parent’s genes (aaBBCc x AABBCC). If you look at each of these, you would see that the offspring (e.g., kids) contain ten different shades of skin color based on the number of capital letters in each genotype.
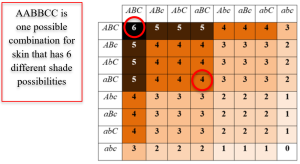
Figure caption: This is an example of skin color polygenic inheritance. Image courtesy of Wikimedia
In some cases, a gene might either be turned on or off depending on the gene with which it is paired. Some genes are considered dominant because they will be expressed regardless if the pairing is heterozygous. Others, termed recessive, are only expressed in the absence of a dominant gene. An example of this can be found in the recessive gene disorder, sickle cell anemia. Sickle cell disease is a condition that is determined by a single pair of genes (one from each parent). The gene that produces healthy round-shaped red blood cells is dominant. The recessive gene causes an abnormality in the shape of red blood cells; they take on a sickle form, which can clog the veins and deprive vital organs of oxygen and increase the risk of stroke. To inherit the disorder a person must receive the recessive gene from both parents. Those who have inherited only one recessive-gene are called carriers and should be unaffected by this recessive trait. Carriers of sickle cell have some red blood cells that take on the c-shaped sickle pattern. Under circumstances of oxygen deprivation, such as high altitudes or physical exertion, carriers for the sickle cell gene may experience some of the symptoms of sickle cell (Berk, 2004). Interestingly, the single gene does not determine the entire story. Sickle cell disease is quite variable in itself. Some variability is genetic (having a thalassemia trait, hemoglobin C) and other variations involve environmental influences. To find out more about more about sickle cell disease, check out the Center for Disease Control (CDC) website.
Chromosomal Abnormalities and Genetic Disorders (Ob 4)
A chromosomal abnormality occurs when a child inherits too many or too few chromosomes. The most common cause of chromosomal abnormalities is the age of the mother. A 20-year-old woman has a 1 in 800 chance of having a child with a common chromosomal abnormality. A woman of 44, however, has a one in 16 chance. It is believed that the problem occurs when the ovum is ripening before ovulation each month. As the mother ages, the ovum is more likely to suffer abnormalities at this time.
Some gametes do not divide evenly when they are forming. Therefore, some cells have more than 46 chromosomes. It is believed that close to half of all zygotes have an odd number of chromosomes. Most of these zygotes fail to develop and are spontaneously aborted by the body. If the abnormal number occurs on pair 21 or 23, however, the individual may have certain physical or other abnormalities. An autosomal chromosome disorders is if individual inherits too many or too few chromosomes not linked to pair 23 (sex chromosomes).
One of the most common chromosomal abnormalities is on pair 21. Trisomy 21 occurs when there are three rather than two chromosomes on pair 21. A person with Down syndrome experiences problems such as intellectual disabilities and certain physical features such as having short fingers and toes, having folds of skin over the eyes, and a protruding tongue. The life expectancy of persons with Down syndrome has increased in recent years. Keep in mind that there is as much variation in people with Down Syndrome as in most populations and those differences need to be recognized and appreciated.
Table Autosomal Chromosomal Abnormalities
| Autosomal Chromosome Disorders: The individual inherits too many or too few chromosomes | Cases per Birth |
|---|---|
| Down Syndrome/Trisomy 21 is caused by an extra chromosome 21 and includes a combination of congenital disabilities. | 1 in 691 1 in 300 births at age 35 |
| Trisomy 13 is caused by an extra chromosome 13. Affected individuals have multiple congenital disabilities and early death. |
1 in 7,906 |
| Trisomy 18 is caused by an extra chromosome 18, and the affected individual also has multiple congenital disabilities and early death | 1 in 3,762 |
Source: Lally & Valentine, 2016
When the abnormality is on pair #23, the result is a sex-linked chromosomal abnormality. A person might have XXY, XYY, XXX, XO, or 45 or 47 chromosomes as a result. Two of the more common sex-linked chromosomal disorders are Turner’s syndrome and Klinefelter’s syndrome. Turner’s syndrome occurs in 1 of every 2,500 live female births (Carroll, 2007) when a sperm fertilizes an ovum which lacks a chromosome with an X chromosome. The resulting zygote has an XO composition. Fertilization by a Y sperm is not viable. Turner syndrome affects cognitive functioning and sexual maturation. The external genitalia appears normal, but breasts and ovaries do not develop fully, and the woman does not menstruate. Turner’s syndrome also results in short stature and other physical characteristics. Klinefelter’s syndrome (XXY) occurs in 1 out of 700 live male births and results when an ovum containing an extra X chromosome is fertilized by a Y sperm. The Y chromosome stimulates the growth of male genitalia, but the extra X chromosome inhibits this development. An individual with Klinefelter’s syndrome has some breast development, infertility (this is the most common cause of infertility in males), and has low levels of testosterone.
Table Sex-Linked Chromosomal Abnormalities
| Sex-Linked Chromosomal Disorders: The disorder occurs on chromosome pair #23 or the sex chromosomes. | Cases per Birth |
| Turner Syndrome is caused when all or part of one of the X chromosomes is lost before or soon after conception due to a random event. The resulting zygote has an XO composition. Turner Syndrome affects cognitive functioning and sexual maturation in girls. Infertility and short stature may be noted. | 1 in 2,500 females |
| Klinefelter Syndrome is caused when an extra X chromosome is present in the cells of a male due to a random event. The Y chromosome stimulates the growth of male genitalia, but the extra X chromosome inhibits this development. The male can have some breast development, infertility, and low levels of testosterone. | 1 in 700 males |
Source: Lally & Valentine, 2016
Most of the known genetic disorders are dominant gene-linked; however, the vast majority of dominant gene-linked disorders are not severe disorders, or if they are, they may still not be debilitating. For example, the majority of those with Tourette’s syndrome suffer only minor tics from time to time and can easily control or cover up their symptoms. Huntington’s Disease is a dominant gene linked disorder that affects the nervous system and is fatal but does not appear until midlife. Recessive gene disorders, such as cystic fibrosis and sickle-cell anemia, are less common but may less likely to be detected as people are unaware that they are carriers of the disease. However, mandatory newborn screening has helped with early diagnosis and treatment for such diseases. If the genes inherited from each parent are the same, the child is homozygous for a particular trait and will inherit the trait. If, however, the child inherits a gene from one parent but not the other, the child is heterozygous, and interaction between the genes will in part determine whether or not that trait is expressed (Berk, 2004).
Table Genetic Disorders
| Recessive Disorders (Homozygous): The individual inherits a gene change from both parents. If the gene is inherited from just one parent, the person is a carrier and does not have the condition. | Cases per Birth |
|---|---|
| Sickle Cell Disease (SCD) is a condition in which the red blood cells in the body are shaped like a sickle (like the letter C) and affect the ability of the blood to transport oxygen. Carries may experience some effects, but do not have the full condition. | 1 in 500 Black births 1 in 36,000 Hispanic births |
| Cystic Fibrosis (CF) is a condition that affects breathing and digestion due to thick mucus building up in the body, especially the lungs and digestive system. In CF, the mucus is thicker than normal and sticky. | 1 in 3,500 |
| Phenylketonuria (PKU) is a metabolic disorder in which the individual cannot metabolize phenylalanine, an amino acid. Left untreated intellectual deficits can occur. PKU is easily detected and is treated with a special diet. | 1 in 10,000 |
| Tay Sachs Disease is caused by enzyme deficiency resulting in the accumulation of lipids in the nerve cells of the brain. This accumulation results in progressive damage to the cells and a decrease in cognitive and physical development. Death typically occurs by age 5. | 1 in 400 1 in 300 American Jew is a carrier 1 in 20 French Canadians are a carrier |
| Albinism is when the individual lacks melanin and possesses little to no pigment in the skin, hair, and eyes. Vision problems can also occur. | Fewer than 20,000 US Cases per year |
| Autosomal Dominant Disorders (Heterozygous): In order to have the disorder, the individual only needs to inherit the gene change from one parent. | Cases per Birth |
| Huntington’s Disease is a condition that affects an individual’s nervous system. Nerve cells become damaged, causing various parts on the brain to deteriorate. The disease affects movement, behavior, and cognition. It is fatal and occurs at midlife. | 1 in 10,000 |
| Tourette Syndrome is a tic disorder which results in uncontrollable motor and vocal ticks as well as body jerking. | 1 in 250 |
| Achondroplasia is the most common form of disproportionate short stature. The individual has abnormal bone growth resulting in short stature, disproportionately short arms and legs, short fingers, a large head, and specific facial features. |
1 in 15,000-40,000 |
Source: Lally & Valentine, 2016
Genetic Counseling (Ob 5)
Genetic counseling refers to a service that assists individuals in identifying, test for, and explain possible genetic conditions that could adversely affect themselves or their offspring (CDC, 2015). The common reasons for genetic counseling include:
- Family history of a genetic condition
- Membership in a particular ethnic group with a higher risk of a genetic condition
- Information regarding the results of genetic testing, including blood tests, amniocentesis, or ultrasounds
- Learning about the chances of having a baby with a genetic condition if the mother is older, has had several miscarriages, has offspring with congenital disabilities, experiences infertility, or has a medical condition
Behavioral Genetics (Ob 3)
Behavioral Genetics is the scientific study of the interplay between the genetic and environmental contributions to behavior. Often referred to as the nature/nurture debate, Gottlieb (1998, 2000, 2002) suggests an analytic framework for this debate that recognizes the interplay between the environment, behavior, and genetic expression. This bidirectional interplay suggests that the environment can affect the expression of genes just as genetic predispositions can impact a person’s potentials.
Additionally, environmental circumstances can trigger symptoms of a genetic disorder. For example, a person who has sickle cell anemia, a recessive gene linked disorder, can experience a sickle cell crisis under conditions of oxygen deprivation. Someone predisposed genetically for type-two diabetes can trigger the disease through poor diet and little exercise.
Research has shown how the environment and genotype interact in several ways. Genotype-Environment Correlations refer to the processes by which genetic factors contribute to variations in the environment (Plomin, DeFries, Knopik, & Niederhiser, 2013). There are three types of genotype-environment correlations:
- Passive genotype-environment correlation occurs when children passively inherit the genes and the environments their family provides. Specific behavioral characteristics, such as being athletically inclined, may run in families. The children have inherited both the genes that would enable success at these activities, and given the environmental encouragement to engage in these actions. For example, this could be demonstrated by a family passes on water skiing skills through both genetics and environmental opportunities.
- Evocative genotype-environment correlation refers to how the social environment reacts to individuals based on their inherited characteristics. For example, whether one has a more outgoing or shy temperament will affect how he or she is treated by others.
- Active genotype-environment correlation occurs when individuals seek out environments that support their genetic tendencies. This is also referred to as niche picking. For example, children who are musically inclined seek out music instruction and opportunities that facilitate their natural musical ability.
Conversely, Genotype-Environment Interactions involve genetic susceptibility to the environment. Adoption studies provide evidence for genotype-environment interactions. For example, the Early Growth and Development Study (Leve, Neiderhiser, Scaramella, & Reiss, 2010) followed 360 adopted children and their adopted and biological parents in a longitudinal study. Results had shown that children whose biological parents exhibited psychopathology, exhibited significantly fewer behavior problems when their adoptive parents used more structured parenting than unstructured. Additionally, elevated psychopathology in adoptive parents increased the risk for the children’s development of behavior problems, but only when the biological parents’ psychopathology was high. Consequently, the results show how environmental effects on behavior differ based on the genotype, especially stressful environments on genetically at-risk children.
Lastly, epigenetics studies modifications in DNA that affect gene expression and are passed on when the cells divide. Environmental factors, such as nutrition, stress, and teratogens are thought to change gene expression by switching genes on and off. These gene changes can then be inherited by daughter cells. This would explain why monozygotic or identical twins may increasingly differ in gene expression with age. For example, Fraga et al. (2005) found that when examining differences in DNA, a group of monozygotic twins were indistinguishable during the early years. However, when the twins were older, there were significant discrepancies in their gene expression, most likely due to different experiences. These differences included susceptibilities to disease and a range of personal characteristics.
Prenatal Development (Ob 6)
Periods of Prenatal Development
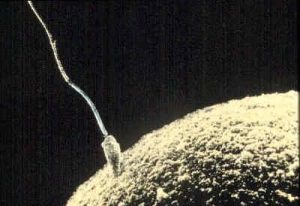
Photo Courtesy of Wikipedia
Now we turn our attention to prenatal development which is divided into three periods: the germinal period, the embryonic period, and the fetal period. While medical doctors refer to trimesters, the three periods of prenatal development are stage based and are not equally distributed as 13-weeks each. Here is an overview of some of the changes that take place during each period.
The Germinal Period
The germinal period starts at conception. At ejaculation, millions of sperm are released into the vagina, but only a few reach the egg, and typically, only one fertilizes the egg. For conception to happen, the ovum or egg needs to be released from the fallopian tube. After the ovum or egg ripens and is released from the ovary, it is drawn into the fallopian tube and in 3 to 4 days, reaches the uterus. The ovum/egg is typically fertilized by the sperm in the fallopian tube and continues its journey to the uterus. Once a single sperm has entered the wall of the egg, the wall becomes hard and prevents other sperm from entering. After the sperm has entered the egg, the tail of the sperm breaks off and the head of the sperm, containing the genetic information from the father, unites with the nucleus of the egg. As a result, a new cell is formed, and this is considered. This cell, containing the combined genetic information from both parents, is referred to as a zygote. The germinal period (about 14 days in length) lasts from conception to implantation of the zygote (fertilized egg) in the lining of the uterus. During this time, the organism begins cell division and growth. After the fourth doubling, differentiation of the cells begins to occur as well. It is estimated that about 60 percent of natural conceptions fail to implant in the uterus. The rate is higher for in vitro conceptions.
During this time, the organism begins cell division through mitosis. After five days of mitosis, there are 100 cells, which is now called a blastocyst. The blastocyst consists of both an inner and an outer group of cells. The inner group of cells or embryonic disk will become the embryo, while the outer group of cells, or trophoblast, becomes the support system which nourishes the developing organism. Other cells develop to form the amniotic sac. The amniotic sac fills with a clear liquid (amniotic fluid) and expands to envelop the developing embryo, which floats within it. This stage ends when the blastocyst fully implants into the uterine wall (U.S. National Library of Medicine, 2015).
Less than one-half of all zygotes survive beyond the first two weeks (Hall, 2004). Some of the reasons for this include the egg and sperm do not join properly. Thus their genetic material does not combine, there is too little or damaged genetic material, the zygote does not replicate, or the blastocyst does not implant into the uterine wall. The failure rate is higher for in vitro conceptions. The figure below illustrates the journey of the ova from its release to its fertilization, cell duplication, and implantation into the uterine lining.
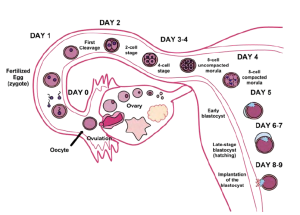
Figure caption: Germinal stage day by day with progression of the fertilized egg. Note that an unfertilized egg would follow the same path but no implantation leading to menses. Image courtesy of WikiCommons
The Embryonic Period
“The body of the unborn baby is more complex than ours. The preborn baby has several extra parts to his body which he needs only so long as he lives inside his mother. He has his own space capsule, the amniotic sac. He has his own lifeline, the umbilical cord, and he has his own root system, the placenta. These all belong to the baby himself, not to his mother. They are all developed from his original cell.”
Day & Liley, The Secret World of a Baby, Random House, 1968, p. 13
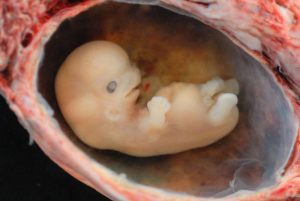
Figure caption: Embryo is 1” long 8-weeks post conception. Photo Courtesy of WikiCommons
This period begins once the multi-cellular organism is implanted in the uterine wall. It lasts from the third through the eighth week after conception. The organism is now called an embryo. The embryo deveops within the amniotic sac, under the lining of the uterus on one side. Now blood vessels grow forming the placenta. The placenta is a structure connected to the uterus that provides nourishment and oxygen from the mother to the developing embryo via the umbilical cord. During this period, cells continue to differentiate and at 22 days after conception the neural tube forms which will become the brain and spinal column. Growth during prenatal development occurs in two primary directions: from head to tail (cephalocaudal development) and from the midline outward (proximodistal development). This means that those structures nearest the head develop before those nearest the feet and those structures nearest the torso develop before those away from the center of the body (such as hands and fingers). The head develops in the fourth week, and the precursor to the heart begins to pulse. In the early stages of the embryonic period, gills and a tail are apparent. However, by the end of this stage, they disappear, and the organism takes on a more human appearance. About 20 percent of organisms fail during the embryonic period, usually due to gross chromosomal abnormalities. As in the case of the germinal period, often the mother does not yet know that she is pregnant. It is during this stage that the major structures of the body are taking form making the embryonic period the time when the organism is most vulnerable to the most significant amount of damage if exposed to harmful substances. (We will look at this in the section on teratology below.) Potential mothers are not often aware of the risks they introduce to the developing child during this time. The embryo is approximately 1 inch in length and weighs about 4 grams at the end of this period (8 weeks post conception). The embryo can move and respond to touch at this time.
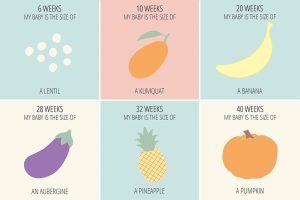
Figure caption: Approximate size of developing fetus analogous to food. Image Courtesy of Lottie Manns
The Fetal Period
The fetal period lasts from the ninth week until birth. During this period, the organism is referred to as a fetus. During this stage, the major structures are continuing to develop. By the 12th week, the fetus has all its body parts including external genitalia. In the following weeks, the fetus will develop hair, nails, teeth and the excretory and digestive systems will continue to develop. At the end of the 12th week, the fetus is about 3 inches long and weighs about 28 grams.
During the 4-6th months, the eyes become more sensitive to light and hearing de, hearing develops. The respiratory system continues to develop. Reflexes such as sucking, swallowing, and hiccupping develop during the 5th month. Cycles of sleep and wakefulness are present at that time as well. The first chance of survival outside the womb, known as the age of viability is reached between 22 and 26 weeks (Moore & Persaud, 1998; Morgan, Goldenberg, & Schulkin, 2008). Many practitioners hesitate to resuscitation before 24 weeks. The majority of the neurons in the brain have developed by 24 weeks although they are still rudimentary and the glial or nurse cells that support neurons continue to grow. At 24 weeks the fetus can feel pain (Royal College of Obstetricians and Gynecologists, 1997).
Between the 7th and 9th months, the fetus is primarily preparing for birth. It is exercising its muscles; its lungs begin to expand and contract. It is developing fat layers under the skin. The fetus gains about 5 pounds and 7 inches during this last trimester of pregnancy which includes a layer of fat gained during the 8th month. This layer of fat serves as insulation and helps the baby regulate body temperature after birth.
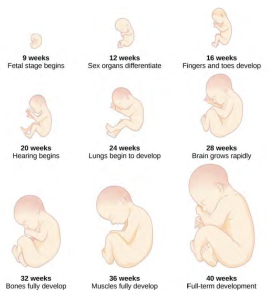
Figure caption: approximate size differences and physical features during pregnancy. Image Courtesy of Cooljargon
Prenatal Brain Development
Fetal brain development begins in the third gestational week with the differentiation of stem cells, which are capable of producing all the different cells that make up the brain (Stiles & Jernigan, 2010). The location of these stem cells in the embryo is referred to as the neural plate. By the end of the third week, two ridges appear along with the neural plate first forming the neural groove and then the neural tube. The open region in the center of the neural tube forms the brain’s ventricles and spinal canal. By the end of the embryonic period, or week eight, the neural tube has further differentiated into the forebrain, midbrain, and hindbrain.
Brain development during the fetal period involves neuron production, migration, and differentiation. From the early fetal period until mid-gestation, most of the 85 billion neurons have been generated, and many have already migrated to their brain positions. Neurogenesis, or the formation of neurons, is primarily completed after 5 months of gestation. One exception is in the hippocampus, which continues to develop neurons throughout life. Neurons that form the neocortex, or the layer of cells that lie on the surface of the brain, migrate to their location in an orderly way. Neural migration is mostly completed by 29 weeks. Once in position neurons begin to produce dendrites and axons that begin to form the neural networks responsible for information processing. Regions of the brain that contain the cell bodies are referred to as the Gray Matter because they look gray in appearance. The axons that form the neural pathways make up the White Matter because they are covered in myelin, a fatty substance that is white in appearance. Myelin aids in both the insulation and efficiency of neural transmission. Although cell differentiation is complete at birth, the growth of dendrites, axons, and synapses continue for years.
Environmental Risks during Prenatal Development (Ob 8)
Teratology
Good prenatal care is essential. The developing child is most at risk for some of the most severe problems during the first three months of development. Unfortunately, this is a time at which most mothers are unaware that they are pregnant. Today, we know many of the factors that can jeopardize the health of the developing child. The study of factors that contribute to congenital disabilities is called teratology. Teratogens are factors that can contribute to congenital disabilities which include some maternal diseases, pollutants, drugs, and alcohol.
Factors influencing prenatal risks: There are several considerations in determining the type and amount of damage that might result from exposure to a particular teratogen (Berger, 2004). These include:
- The timing of the exposure: Structures in the body are vulnerable to the most severe damage when they are forming. If a substance is introduced during a particular structure’s critical period (time of development), the damage to that structure may be more significant. For example, the ears and arms reach their critical periods at about six weeks after conception. If a mother exposes the embryo to certain substances during this period, the arms and ears may be malformed.
- The amount of exposure: Some substances are not harmful unless the amounts reach a certain level. The critical level depends in part on the size and metabolism of the mother.
- Dose-response relation: The higher the exposure to the potential teratogen, the more likely it is that the fetus will suffer damage and the more severe the damage is likely to be with greater exposure.
- Genetics: Genetic makeup also plays a role in the impact a particular teratogen might have on the child. This is suggested by fraternal twin studies who are exposed to the same prenatal environment, yet do not experience the same teratogenic effects. The genetic makeup of the mother can also have an effect; some mothers may be more resistant to teratogenic effects than others.
- Being male or female: Males are more likely to experience damage due to teratogens than are females. It is believed that the Y chromosome, which contains fewer genes than the X, may have an impact.
Critical Periods of Development
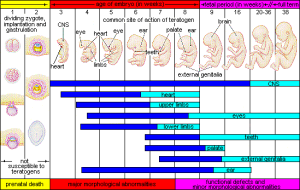
Figure caption: Critical Periods of Prenatal Development. The three periods of development are summarized along with major growth phases for different parts of the body. Note that in the first 2 weeks, the developing zygote is not susceptible to teratogens. Areas in blue indicate major development formation (first shaded area for each body part). Image courtesy of WikiCommons
The figure above summarizes the critical periods of development during the 3 periods of prenatal development. You will notice that during the germinal stage (first part of graph), teratogens are not an issue with development (rather risk is more genetic). The next stage (embryonic) is where the majority of development occurs. The darker areas for each show critical development and the lighter areas indicate more refinement of that organ/body part.
A look at some teratogens
Alcohol is one of the most commonly used teratogens is alcohol, and because half of all pregnancies in the United States are unplanned, it is recommended that women of childbearing age take great caution against drinking alcohol when not using birth control or when pregnant (Surgeon General’s Advisory on Alcohol Use During Pregnancy, 2005). Alcohol consumption, particularly during the second month of prenatal development but at any point during pregnancy may lead to neurocognitive and behavioral difficulties that can last a lifetime. Binge drinking (five or more on a single occasion) or seven or more drinks during a single week place a child at risk.
In extreme cases, alcohol consumption can lead to fetal death, but more frequently it can result in fetal alcohol spectrum disorders (FASD), an umbrella term for a range of effects of exposure and replaces the term fetal alcohol syndrome. It is preferred because it recognizes that symptoms occur on a spectrum and that all individuals do not have the same characteristics. The most severe form of FASD is Fetal Alcohol Syndrome (FAS). Children with FAS share certain physical features such as flat noses, small eye holes, and small heads (see Figure). Cognitively, these children have poor judgment, poor impulse control, higher rates of ADHD, learning issues, and lower IQ scores. These developmental problems and delays persist into adulthood (Streissguth, Barr, Kogan, & Bookstein, 1996) and can include criminal behavior, psychiatric problems, and unemployment (CDC, 2016a). Based on animal studies, it has been hypothesized that a mother’s alcohol consumption during pregnancy may predispose her child to like alcohol (Youngentob, Molina, Spear, & Youngentob, 2007).
- The prevalence of FASD in the US is estimated to be between one and five percent, in otherwords, in 11 and 50 cases per 1000 children (May, Chambers, Kalberg, 2018).
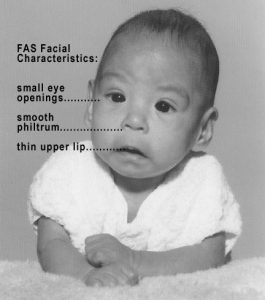
Figure caption: An infant with FAS. Photo Courtesy of WikiCommons
| Facial Feature | Potential Effect of Fetal Alcohol Syndrome |
|---|---|
| Head size | Below-average head circumference |
| Eyes | Smaller than average eye-opening, skin folds at corners of eyes |
| Nose | Low nasal bridge, short nose |
| Midface | Smaller than average midface size |
| Lip and philtrum | Thin upper lip, indistinct philtrum |
Table Summary: features of FAS. Source: https://legacy.cnx.org/content/m49112/latest/?collection=col11629/latest
Tobacco is the second most widely used teratogen, and the number of adolescent females who smoke is increasing. In fact, among adolescents, females are just as likely to smoke as are males. Tobacco use during pregnancy has been associated with low birth weight, placenta previa, preterm delivery, fetal growth restriction, and sudden infant death syndrome (Center for Disease Control, 2004). According to Tong et al. (2013) in conjunction with the Centers for Disease Control and Prevention, data from 27 sites in 2010 representing 52% of live births, showed that among women with recent live births:
- About 23% reported smoking in the 3 months prior to pregnancy.
- Almost 11% reported smoking during pregnancy.
- More than half (54.3%) reported that they quit smoking by the last 3 months of pregnancy.
- Almost 16% reported smoking after delivery.
When comparing the ages of women who smoked:
- Women < 20, 13.6% smoked during pregnancy
- Women 20–24, 17.6% smoked during pregnancy
- Women 25–34, 8.8% smoked during pregnancy
- Women ≥ 35, 5.7% smoked during pregnancy
The findings among racial and ethnic groups indicated that smoking during pregnancy was highest among American Indians/Alaska Natives (26.0%) and lowest among Asians/Pacific Islanders (2.1%).
When a pregnant woman smokes the fetus is exposed to dangerous chemicals including nicotine, carbon monoxide, and tar, which lessen the amount of oxygen available to the fetus. Oxygen is essential for overall growth and development. Tobacco use during pregnancy has been associated with low birth weight, ectopic pregnancy (fertilized egg implants itself outside of the uterus), placenta previa (placenta lies low in the uterus and covers all or part of the cervix), placental abruption (placenta separates prematurely from the uterine wall), preterm delivery, stillbirth, fetal growth restriction, sudden infant death syndrome (SIDS), birth defects, learning disabilities, and early puberty in girls (Center for Disease Control, 2015d). A woman being Exposed to second-hand smoke during pregnancy has also been linked to low-birth-weight infants.

Photo Courtesy of Amy Shephard
Prescription/Over-the-counter Drugs are other possible teratogens. About 70% of pregnant women take at least one prescription drug (March of Dimes, 2016e). A woman should not be taking any prescription drug during pregnancy unless it was prescribed by a health care provider who knows she is pregnant. Some prescription drugs can cause congenital disabilities, problems in overall health, and development of the fetus. Over-the-counter drugs are also a concern during the prenatal period because they may cause specific health problems. For example, the pain reliever ibuprofen can cause severe blood flow problems to the fetus during the last three months.
Common illicit drugs include cocaine, ecstasy, heroin, marijuana, and prescription drugs that are abused. It is difficult to ultimately determine the effects of a particular illicit drug on a developing child because most mothers who use, use more than one substance and have other unhealthy behaviors. These include smoking, drinking alcohol, not eating healthy meals, and being more likely to get a sexually transmitted disease. However, several problems seem clear. The use of cocaine is connected with low birth weight, stillbirths, and spontaneous abortion. Heavy marijuana use is associated with problems in brain development (March of Dimes, 2016c). If a baby’s mother used an addictive drug during pregnancy, that baby can get addicted to the drug before birth and go through drug withdrawal after birth, also known as Neonatal Abstinence Syndrome (March of Dimes, 2015d). Other complications of illicit drug use include premature birth, smaller than average head size, congenital disabilities, heart defects, and infections. Additionally, babies born to mothers who use drugs may have problems later in life, including learning and behavior difficulties, slower than average growth, and die from Sudden Infant Death Syndrome. Children of substance abusing parents are also considered at high risk for a range of biological, developmental, academic, and behavioral problems, including developing substance abuse problems of their own (Conners et al., 2003).
Pollutants are another possible teratogen. There are more than 83,000 chemicals used in the United States with little information on the effects of them during pregnancy (March of Dimes, 2016b). An environmental pollutant of significant concern is lead poisoning, which is connected with low birth weight and slowed neurological development. The chemicals in certain pesticides are also potentially damaging and may lead to congenital disabilities, learning problems, low birth weight, miscarriage, and premature birth (March of Dimes, 2014). Prenatal exposure to bisphenol A (BPA), a chemical commonly used in plastics and food and beverage containers, may disrupt the action of specific genes contributing to certain congenital disabilities (March of Dimes, 2016b). Radiation is another environmental hazard. If a mother is exposed to radiation, it can get into the bloodstream and pass through the umbilical cord to the baby. Radiation can also build up in body areas close to the uterus, such as the bladder. Exposure to radiation can slow the baby’s growth, cause congenital disabilities, affect brain development, cause cancer, and result in a miscarriage. Mercury, a heavy metal, can cause brain damage and affect the baby’s hearing and vision. This is why women are cautioned about the amount and type of fish they consume during pregnancy.
Toxoplasmosis is a concern for congenital disabilities. The tiny parasite, Toxoplasma gondii, causes an infection called Toxoplasmosis. According to the March of Dimes (2012d), Toxoplasma gondii infects more than 60 million people in the United States. A healthy immune system can keep the parasite at bay producing no symptoms, so most people do not know they are infected. As routine prenatal screening frequently does not test for the presence of this parasite, pregnant women may want to talk to their health-care provider about being tested. Toxoplasmosis can cause premature birth, stillbirth, and can result in congenital disabilities to the eyes and brain. While most babies born with this infection show no symptoms, ten percent may experience eye infections, enlarged liver and spleen, jaundice, and pneumonia. To avoid being infected, women should avoid eating undercooked or raw meat and unwashed fruits and vegetables, touching cooking utensils that touched raw meat or unwashed fruits and vegetables, and touching cat feces, soil or sand. If women think they may have been infected during pregnancy, they should have their baby tested.

Figure caption: Changing a cat litter box may expose individual to toxoplasmosis. Photo courtesy of Flickr.
Sexually Transmitted Diseases such as Gonorrhea, syphilis, and chlamydia can be passed to the fetus by an infected mother. Mothers should be tested as early as possible to minimize the risk of spreading these infections to their unborn child. Additionally, the earlier the treatment begins, the better the health outcomes for mother and baby (CDC, 2016d). Sexually transmitted diseases (STDs) can cause premature birth, premature rupture of the amniotic sac, an ectopic pregnancy, congenital disabilities, miscarriage, and stillbirths (March of Dimes, 2013). Most babies become infected with STDs while passing through the birth canal during delivery, but some STDs can cross the placenta and infect the developing fetus.
Human Immunodeficiency Virus (HIV) is one of the most potentially devastating teratogens. HIV and Acquired Immune Deficiency Syndrome (AIDS) are leading causes of illness and death in the United States (Health Resources and Services Administration, 2015). One of the main ways children under age 13 become infected with HIV is via mother-to-child transmission of the virus prenatally, during labor, or by breastfeeding (CDC, 2016c). Some measures can be taken to lower the chance the child will contract the disease. HIV positive mothers who take antiviral medications during their pregnancy significantly reduce the chance of passing the virus to the fetus. The risk of transmission is less than 2 percent; in contrast, it is 25 percent if the mother does not take antiretroviral drugs (CDC, 2016b). However, the long-term risks of prenatal exposure to the medication are not known. It is recommended that women with HIV deliver the child by C-section and that after birth they avoid breastfeeding.
Rubella, also called German measles, is an infection that causes mild flu-like symptoms and a rash on the skin. However, only about half of children infected have these symptoms, while others have no symptoms (March of Dimes, 2012a). Rubella has been associated with a number of congenital disabilities. If the mother contracts the disease during the first three months of pregnancy, damage can occur in the eyes, ears, heart, or brain of the unborn child. Deafness is almost certain if the mother has German measles before the 11th week of prenatal development and can also cause brain damage. Women in the United States are much less likely to be afflicted with rubella because most women received childhood vaccinations that protect her from the disease.
Maternal Factors
Mothers over 35: Most women over 35 who become pregnant are in good health and have healthy pregnancies. However, according to the March of Dimes (2016d), women over age 35 are more likely to have an increased risk of:
- Fertility problems
- High blood pressure
- Diabetes
- Miscarriages
- Placenta Previa
- Cesarean section
- Premature birth
- Stillbirth
- A baby with a genetic disorder or other congenital disabilities
Because a woman is born with all her eggs, environmental teratogens can affect the quality of the eggs as women get older. Also, a woman’s reproductive system ages which can adversely affect the pregnancy. Some women over 35 choose special prenatal screening tests, such as a maternal blood screening, to determine if there are any health risks for the baby.
Although there are medical concerns associated with having a child later in life, there are also many positive consequences to being a more mature parent. Older parents are more confident, less stressed, and typically married providing family stability. Their children perform better on math and reading tests, and they are less prone to injuries or emotional troubles (Albert, 2013). Women who choose to wait are often well educated and lead healthy lives. According to Gregory (2007), older women are more stable, demonstrate a stronger family focus, possess greater self-confidence, and have more money. Having a child later in one’s career equals overall higher wages. In fact, for every year a woman delays motherhood, she makes 9% more in lifetime earnings. Lastly, women who delay having children live longer. Sun et al. (2015) found that women who had their last child after the age of 33 doubled their chances of living to age 95 or older than women who had their last child before their 30th birthday. A woman’s natural ability to have a child at a later age indicates that her reproductive system is aging slowly, and consequently so is the rest of her body.
Teenage Pregnancy: A teenage mother is at a higher risk for having pregnancy complications including anemia, and high blood pressure. These risks are even more significant for those under age 15. Infants born to teenage mothers have a higher risk of being premature and having low birth weight or other serious health problems. Reasons for these health issues include that teenagers are the least likely of all age groups to get early and regular prenatal care. Additionally, they may engage in harmful behaviors including eating unhealthy food, smoking, drinking alcohol, and taking drugs. Additional concerns for teenagers are repeat births. About 25% of teen mothers under age 18 have a second baby within two years after the first baby’s birth.
Low Socioeconomic Status: Low SES contributes to lack of access to prenatal care, proper nutrition, and often, social support. There is a negative association between low SES and pregnancy complications (Parker, Schoendorf, & Kiely, 1994). Research has revealed that low SES is associated with pregnancy complications such as abortion, preterm delivery, preeclampsia, eclampsia, and gestational diabetes (Kim et al., 2018). It is unknown the extent to which this is attributed to inadequate prenatal care. However, low SES has several challenges for receiving adequate prenatal care. First, occupational factors, such as long working hours and physical exertion may prevent adequate prenatal visits, and extended working hours or occupational fatigue is associated with preterm birth and preeclampsia (Kim et al., 2008). Second, other economic factors like costs of transportation to the hospital and the opportunity cost of receiving medical care may be a sufficient burden that restricts prenatal care in pregnant women with low SES (Kim et al., 2018). Third, the low educational level is related to the probability of seeking antenatal care inappropriately (Kim et al., 2018). Women with a low SES are also at risk for feeling demoralized and depressed.
Gestational Diabetes: Seven percent of pregnant women develop gestational diabetes (March of Dimes, 2015b). Diabetes is a condition where the body has too much glucose in the bloodstream. Most pregnant women have their glucose level tested at 24 to 28 weeks of pregnancy. Gestational diabetes usually goes away after the mother gives birth, but it might indicate a risk of developing diabetes later in life. If untreated, gestational diabetes can cause premature birth, stillbirth, the baby having breathing problems at birth, jaundice, or low blood sugar. Babies born to mothers with gestational diabetes can also be considerably heavier (more than 9 pounds) making the labor and birth process more difficult. For expectant mothers, untreated, gestational diabetes can cause preeclampsia (high blood pressure and signs that the liver and kidneys may not be working correctly) discussed later in the chapter. Risk factors for gestational diabetes include age (being over age 25), being overweight or gaining too much weight during pregnancy, family history of diabetes, having had gestational diabetes with a prior pregnancy, and race and ethnicity (African-American, Native American, Hispanic, Asian, or Pacific Islander have a higher risk). Eating healthy and maintaining a healthy weight during pregnancy can reduce the chance of gestational diabetes. Women who already have diabetes and become pregnant need to attend all their prenatal care visits, and follow the same advice as those for women with gestational diabetes as the risk of preeclampsia, premature birth, congenital disabilities, and stillbirth are the same.
High Blood Pressure (Hypertension): Hypertension is a condition in which the pressure against the wall of the arteries becomes too high. There are two types of high blood pressure during pregnancy, gestational and chronic. Gestational hypertension only occurs during pregnancy and goes away after birth. Chronic high blood pressure refers to women who already had hypertension before the pregnancy or to those who developed it during pregnancy, and it did not go away after birth. According to the March of Dimes (2015c), about 8 in every 100 pregnant women have high blood pressure. High blood pressure during pregnancy can cause premature birth, and low birth weight (under 5.5 pounds), placental abruption, and mothers can develop preeclampsia.
Figure caption: Blood pressure should be monitored during pregnancy. Photo courtesy of Flickr
Rh Disease: Rh is a protein found in the blood. Most people are Rh positive, meaning they have this protein. Some people are Rh negative, meaning this protein is absent. Mothers who are Rh negative are at risk of having a baby with a form of anemia called Rh disease (March of Dimes, 2009). A father who is Rh-positive and mother who is Rh-negative can conceive a baby who is Rh-positive. Some of the fetus’s blood cells may get into the mother’s bloodstream, and her immune system is unable to recognize the Rh factor. The immune system starts to produce antibodies to fight off what it thinks is a foreign invader. Once her body produces immunity, the antibodies can cross the placenta and start to destroy the red blood cells of the developing fetus. As this process takes time, often the first Rh-positive baby is not harmed, but as the mother’s body will continue to produce antibodies to the Rh factor across her lifetime, subsequent pregnancies can pose a higher risk for an Rh-positive baby. In the newborn, Rh disease can lead to jaundice, anemia, heart failure, brain damage, and death.
Weight Gain during Pregnancy: According to March of Dimes (2016f) during pregnancy most women need only an additional 300 calories per day to aid in the growth of the fetus. Gaining too little or too much weight during pregnancy can be harmful. Women who gain too little may have a baby who is low birth weight, while those who gain too much are likely to have a premature or large baby. There is also a higher risk for the mother developing preeclampsia and diabetes, which can cause further problems during pregnancy. Guidelines healthy weight gain during pregnancy vary by a mother’s weight before pregnancy. Putting on the weight slowly is best. Mothers who are concerned about their weight gain should talk to their health care provider.
Stress: Feeling stressed is common during pregnancy, but high levels of stress can cause complications including having a premature baby or a low-birthweight baby. Babies born early or too small are at an increased risk for health problems. Stress-related hormones may cause these complications by affecting a woman’s immune systems resulting in an infection and premature birth. Additionally, some women deal with stress by smoking, drinking alcohol, or taking drugs, which can lead to problems in the pregnancy. High levels of stress in pregnancy have also been correlated with problems in the baby’s brain development and immune system functioning, as well as childhood problems such as trouble paying attention and being afraid (March of Dimes, 2012b).
Stress during the pregnancy may also be related to labor complications and the parent-child attachment. After a stressful pregnancy, a woman might feel less prepared and more anxious about birth, experience more pain and less support during labor, and consequently struggle more with postpartum recovery, breastfeeding, bonding, and distressed mood (Saxby, 2017). Prenatal stress and anxiety have been associated with obstetric complications, preterm labor onset, risk of C-section birth, and greater use of pain medication during labor (Alder, Fink, Bitzer, Hösli, & Holzgreve, 2007; Saunders, Lobel, Veloso, & Meyer, 2006).
Depression: Depression is a medical condition in which feelings of sadness, worthlessness, guilt, and fatigue interfere with one’s daily functioning. Depression can occur before, during, or after pregnancy, and 1 in 7 women is treated for depression sometime between the year before pregnancy and year after pregnancy (March of Dimes, 2015a). Women who have experienced depression previously are more likely to have depression during pregnancy. Consequences of depression include the baby being born premature, having a low birth weight, being more irritable, less active, less attentive, and having fewer facial expressions. About 13% of pregnant women take an antidepressant during pregnancy. It is essential that women taking antidepressants during pregnancy discuss the medication with a health care provider as some medications can cause harm to the developing organism.

Image courtesy of Pixabay
Depression is not the same as the Baby Blues. The Baby Blues are feelings of sadness that occur 3 to 5 days after having a baby and typically disappear usually within 10 days of the birth. New mothers may have trouble sleeping, be moody, and feel let-down from the birthing experience. According to the Diagnostic and Statistical Manual of Mental Disorders-5th edition (DSM-V), (American Psychiatric Association, 2013), the peripartum onset of depression, also known as Postpartum Depression, is a type of depression that occurs during pregnancy or in the four weeks following pregnancy. Approximately 1 out of 8 women experience postpartum depression. Changing hormone levels are thought to be a factor in its occurrence. However, risk factors include having depression previously, a family history of depression, being younger than 20, experiencing stress, and substance use.
Peripartum-onset mood disorders, both depression, and mania can present with or without psychotic features. Hallucinations and delusions are associated with postpartum psychotic episodes and have included command hallucinations to kill the infant or delusions that the infant is possessed. Psychotic features occur in approximately 1 in 500 to 1 in 1,000 deliveries, and the risk is higher for women with previous postpartum mood episodes (American Psychiatric Association, 2013).
Paternal Impact: The age of fathers at the time of conception is also an important factor in health risks for children. According to Nippoldt (2015) offspring of men over 40 face an increased risk of miscarriages, autism, congenital disabilities, achondroplasia (bone growth disorder), and schizophrenia. These increased health risks are thought to be due to accumulated chromosomal aberrations and mutations during the maturation of sperm cells in older men (Bray, Gunnell, & Smith, 2006). However, like older women, the overall risks are small.
Also, men are more likely than women to work in occupations where hazardous chemicals, many of which have teratogenic effects or may cause genetic mutations, are used (Cordier, 2008). These may include petrochemicals, lead, and pesticides that can cause abnormal sperm and lead to miscarriages or diseases. Men are also more likely to be a source of second-hand smoke for their developing offspring. As noted earlier, smoking by either the mother or around the mother can hinder fetal development.
Pregnancy and Childbirth (Ob 7)
Prenatal Assessment
Several assessments are suggested to women as part of their routine prenatal care to find conditions that may increase the risk of complications for the mother and fetus (Eisenberg, Murkoff, & Hathaway, 1996). These can include blood and urine analyses and screening and diagnostic tests for congenital disabilities.
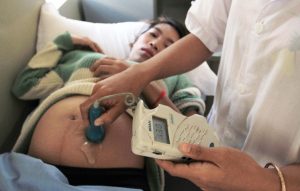
Figure caption: medial staff checking heartbeat of fetus. Photo Courtesy of World Bank Photo Collection
Ultrasound is one of the primary screening tests done in combination with blood tests. The ultrasound is a test in which sound waves are used to examine the fetus. There are two general types. Transvaginal ultrasounds are used early pregnancy, while transabdominal ultrasounds are more common and used after 10 weeks of pregnancy (typically, 16 to 20 weeks). Ultrasounds are used to check the fetus for defects or problems. It can also find out the age of the fetus, location of the placenta, fetal position, movement, breathing, and heart rate, amount of amniotic fluid in the uterus, and the number of fetuses. Most women have at least one ultrasound during pregnancy, but if problems are noted, additional ultrasounds may be recommended.
When a diagnosis of congenital disability is necessary, ultrasounds help guide the more invasive diagnostic tests of amniocentesis and chorionic villus sampling. Amniocentesis is a procedure in which a needle is used to withdraw a small amount of amniotic fluid and cells from the sac surrounding the fetus and later tested. Chorionic Villus Sampling is a procedure in which a small sample of cells is taken from the placenta and tested. Both amniocentesis and chorionic villus sampling have a risk of miscarriage, and consequently, they are not done routinely.
Complications of Pregnancy and Delivery
Minor complications: There are several common side effects of pregnancy. Not everyone experiences all of these nor to the same degree. Moreover, although they are considered “minor,” this is not to say that these problems are potentially very uncomfortable. These side effects include nausea (particularly during the first 3-4 months of pregnancy as a result of higher levels of estrogen in the system), heartburn, gas, hemorrhoids, backache, leg cramps, insomnia, constipation, shortness of breath or varicose veins (as a result of carrying a heavy load on the abdomen). Some may complain about breast tenderness as colostrum, the first breast milk rich in nutrients is produced during pregnancy. All of these complications may subside or disappear after delivery.
Major Complications: The following are some severe complications of pregnancy which can pose health risks to mother and child and that often require hospitalization. Ectopic Pregnancy occurs when the zygote becomes attached to the fallopian tube before reaching the uterus. About 1 in 50 pregnancies in the United States are tubal pregnancies, and this number has been increasing because of the higher rates of pelvic inflammatory disease and Chlamydia (Carroll, 2007). Abdominal pain, vaginal bleeding, nausea, and fainting are symptoms of ectopic pregnancy.
Preeclampsia, also known as Toxemia, is characterized by a sharp rise in blood pressure, leakage of protein into the urine as a result of kidney problems, and swelling of the hands, feet, and face during the third trimester of pregnancy. Preeclampsia is the most common complication of pregnancy. It is estimated to affect 5% to 10% of all pregnancies globally and accounts for 40% to 60% of maternal deaths in developing countries (National Institute of Child Health and Human Development, 2013). Rates are lower in the United States, and preeclampsia affects about 3% to 5% of pregnant women. Preeclampsia occurs most frequently in first pregnancies, and it is more common in women who are obese, have diabetes, or are carrying twins. When preeclampsia causes seizures, the condition is known as eclampsia, which is the second leading cause of maternal death in the United States. Preeclampsia is also a leading cause of fetal complications, which include low birth weight, premature birth, and stillbirth. Treatment is typically bed rest and sometimes medication. If this treatment is ineffective, labor may be induced.
Maternal Mortality: Approximately 1000 women die in childbirth around the world each day (World Health Organization, 2010). Rates are highest in Sub-Saharan Africa and South Asia although there has been a substantial decrease in these rates. The campaign to make childbirth safe for everyone has led to the development of clinics accessible to those living in more isolated areas and training more midwives to assist in childbirth.
Spontaneous abortion is experienced in an estimated 20-40 percent of undiagnosed pregnancies and another 10 percent of diagnosed pregnancy. Usually, the body aborts due to chromosomal abnormalities, and this typically happens before the 12th week of pregnancy. Cramping and bleeding result and regular periods return after several months. Some women are more likely to have repeated miscarriages due to chromosomal, amniotic, or hormonal problems; but miscarriage can also be a result of defective sperm (Carroll et al., 2003).
Infant anoxia: During delivery one major complication possible for the baby is anoxia. Anoxia is a temporary lack of oxygen to the brain. Difficulty during delivery may lead to anoxia which can result in brain damage or severe cases, death. Babies who suffer both low birth weight and anoxia are more likely to suffer learning disabilities later in life as well.
Chapter 3 Key terms
| chromosome | chromosomal abnormalities |
| DNA (deoxyribonucleic acid) | down syndrome |
| gene | fetal alcohol spectrum disorder |
| epigenetics | zygote |
| sex chromosomes | germinal period |
| gametes | blastocyst |
| ovum | embryonic period |
| meiosis | fetal period |
| placenta | low birth weight |
| umbilical cord | preterm |
| amniotic sac | infant mortality |
| teratogen | |
| critical periods |
stages of birth |
| genetic disorders |
Media Attributions
- Chapter_3_Heredity_Prenatal_Development_And_Birth_01
- Chapter_3_Heredity_Prenatal_Development_And_Birth_02
- Chapter_3_Heredity_Prenatal_Development_And_Birth_03
- Chapter_1_Intro_to_Lifespan_Growth_and_Developmental_Include_V3_03
- Chapter_3_Heredity_Prenatal_Development_And_Birth_04
- Chapter_3_Heredity_Prenatal_Development_And_Birth_05
- Chapter_3_Heredity_Prenatal_Development_And_Birth__Include_V3_01
- chapter_3_Heredity_Prenatal_Development_And_Birth_07
- Chapter_3_Heredity_Prenatal_Development_And_Birth_08
- chapter_3_Heredity_Prenatal_Development_And_Birth_09
- chapter_3_Heredity_Prenatal_Development_And_Birth__Include_V3_02
- Chapter_3_Heredity_Prenatal_Development_And_Birth_10
- chapter_3_Heredity_Prenatal_Development_And_Birth_11
- chapter_3_Heredity_Prenatal_Development_And_Birth_12
- chapter_3_Heredity_Prenatal_Development_And_Birth_13
- chapter_3_Heredity_Prenatal_Development_And_Birth__Include_V3_03
- chapter_3_Heredity_Prenatal_Development_And_Birth__Include_V3_04
- Chapter_3_Heredity_Prenatal_Development_And_Birth__Include_V3_05
- chapter_3_Heredity_Prenatal_Development_And_Birth_15

